
Journal of Vita Columbia Volume 2 Issue 1 – Clinical Nutrition
Vitamin A: A Crucial Micronutrient for Immunity, Vision, Reproduction
January 5th, 2022
Authors:
Blessy K Joy, MBBS
Kevin Joy, BSc, DCh
Wafaa Chorfi, MD
How to cite this article:
Joy BK, Joy K, Chorfi W. Vitamin A: A Crucial Micronutrient for Immunity, Vision, Reproduction. 2022. Journal of Vita Columbia. 2(1).
Abstract:
Retinol, commonly known as vitamin A, is a crucial micronutrient needed for multiple functions in the body including immune functions, vision, and reproduction. Vitamin A comes from both animal and plant sources as well as from fortified cereals. It is absorbed with fat micelles and stored in liver as retinol, and has several therapeutic uses including Covid-19 treatment. This article also covers the deficiency and toxicity of vitamin A and how to treat them. It also showcases some of the controversies of vitamin A and its relationship with certain cancers and cardiovascular diseases, as well as the future scope in research surrounding vitamin A.
Introduction:
Vitamin A is an important micronutrient with antioxidant properties which circulates as Retinol in our body. These are fat soluble retinoids including retinol, retinal and the esterified form [1]. The major sources are vegetarian and animal sources and can be synthesized in the labs as synthetic retinoids for medical uses. It is absorbed from the duodenum and proximal jejunum along with fat molecules and transported with retinol-binding protein (RBP) to liver for storage as retinol [38]. The active form of vitamin A is retinal and retinoic acid, the latter is bound to albumin for transport. Like any other nutrient vitamin A can also cause deficiency and toxicity, both of which must be managed accordingly. It is also used in the treatment of many diseases including measles, HIV, infertility, blindness, and even COVID-19.
Functions of Vitamin A:
-
Immune functions
Vitamin A is involved in production of leukocytes including natural killer cells, macrophages, and the mucosal barriers. Deficiency of vitamin A can lead to increased susceptibility to infections.
- Vision
Vitamin A is a crucial factor in forming rhodopsin which is a protein that absorbs light in the retinal receptors [2], and to some extent in color vision. Deficiency of rhodopsin can cause night blindness and later complete blindness.
-
Reproduction
In males it is involved in spermatogenesis and oogenesis in females. It plays an important role in placental development, fetal and embryonic growth [38]. Deficiency of vitamin A can lead to infertility and is critical for adequate fetal growth.
Metabolism of Vitamin A:
Vitamin A is a fat-soluble vitamin. Hence, different forms of vitamin A present in food are solubilized into micelles in the duodenal lumen and proximal jejunum from which they are absorbed. Inside the body, both forms of vitamin A are converted into retinol, which is the measurable form. However, the active form of vitamin A is retinal and retinoic acid.
Vitamin A is primarily stored in the liver as retinyl esters. A downside to this is plasma retinol levels will decrease only once the liver stores are depleted. So, dietary deficiency cannot be measured in the early stages. However, liver stores of vitamin A can be measured indirectly through the dose response test. In this test plasma retinol levels are measured before and after the administration of a small amount of vitamin A. An increase of 20% or above in serum retinol levels shows vitamin A deficiency [3]. For regular clinical practice, serum retinol levels are enough.
Sources of Vitamin A:
2 types of vitamin A that are found in human diet which includes [3]:
- Preformed vitamin A
- Provitamin A
Preformed vitamin A consists of retinyl ester and retinol. Major sources include animal products like fish, and meat specifically liver. Among provitamin A carotenoids the most important one is beta-carotene and is mainly obtained from plant sources. Provitamin A is converted into its active form, i.e., retinal, and retinoic acid within our body [3]. Other sources of vitamin A include fortified cereals.
| Table 1. Vitamin A Content In Selected Foods [39, 40] | |||||
| FOOD | RAE* | FOOD | RAE* | ||
| VEGETABLE | FRUITS | ||||
| Amaranth – raw leaf |
900-1,543 |
Apple | 7 | ||
| Beet | 487 | Apricot (dried) | 210-1,090 | ||
| Broccoli, boiled | 60 |
Avocado |
10-88 | ||
| Cabbage | Green | 10 | Banana | 10-21 | |
| Red | 3 |
Blueberry |
10-28 | ||
| Carrot | 1,200 |
Mandarin |
42 | ||
| Dandelion | 1,200-1367 | Mango | Ripe | 118-400 | |
| Kale | 150-1,263 | Unripe | 10 | ||
| Lettuce | 325 | Dried | 733-887 | ||
| Okra | 121 | Raspberry | 10 | ||
| Potato | Trace – 3 | Watermelon | 8-58 | ||
| Pumpkin | 166 | OILS | |||
| Spinach, boiled | 573 | Coconut oil | 0 | ||
| Rice – parboiled | 0 | Olive oil | 4 | ||
| Sweet potato (boiled) | 291 | Red palm oil | 2,035-24,647 | ||
| Taro (leaf, boiled) | 783 | FISH | |||
| MILK AND EGG | Oyster | 90-96 | |||
| Buffalo milk | 64 | Salmon, cooked | 70 | ||
| Cow milk | 29-38 | Tuna | 80-830 | ||
| Goat milk | 19-71 | MEAT | |||
| Yoghurt, plain | 23 | Beef | 25 | ||
| Chicken egg | 260 |
Chicken |
10-74 | ||
| Duck egg | 740 | Goat |
0 |
||
|
*All values are for raw food unless specified. Total vitamin A activity (RAE) per 100g of edible portion |
|||||
Clinical Uses of Vitamin A:
Vitamin A has several therapeutic implications.
- Measles – Administering vitamin A to children with measles has shown to reduce the complications and mortality associated with the disease [41].
- Dermatology – Synthetic retinoids like isotretinoin is used in treating conditions like psoriasis, hyperkeratosis, skin cancer etc. It is also used in the treatment of acne both topically and systemically. Due to teratogenicity of vitamin A, precaution is taken not to get pregnant while on retinoic acid [42].
- Atherosclerosis – Attributable to the antioxidative properties of retinol, it may be useful in preventing cardiovascular disease. But this is controversial as certain studies have pointed that it may increase the likelihood of lung cancer and cardiovascular diseases [15].
- Acute promyelocyte leukemia (a subgroup of Acute Myelocytic Leukemia) – All Trans Retinoic Acid or tretinoin, a metabolite of vitamin A, is used in treatment of acute promyelocytic leukemia in conjunction with chemotherapy [43].
- Very low birth weight infants – Studies have shown a decline in the incidence of bronchopulmonary dysplasia in very low birthweight infants after supplementing with vitamin A [16].
- Age Related Macular Degeneration – The probable etiology for ARMD is oxidative stress, for which reason Vitamin A is beneficial in the treatment and prevention of ARMD by giving supplements containing carotenoids [17].
Recommended Daily Allowance (RDA) of Vitamin A:
The recommended daily allowance for vitamin A is measured as retinol activity equivalents (RAE).
1 RAE = 1 mcg retinol or 3.3 IU [5]
|
Table 2 – Showing Vitamin A Requirements for Different Age Groups [3] |
||
|
Age group |
RDA/AI* (microgram) |
UL (maximum daily intake) |
| Infants | ||
|
0-6 months |
400* |
600 |
|
7-12 months |
500* |
600 |
|
Children |
||
| 1-3 years |
300 |
600 |
| 4-8 years |
400 |
900 |
| Males | ||
| 9-13 years |
600 |
1700 |
| 14-18 years |
900 |
2800 |
| >19 years |
900 |
3000 |
|
Females |
||
| 9-13 years |
600 |
1700 |
| 14-18 years |
700 |
2800 |
| >19 years |
700 |
3000 |
| Pregnancy | ||
| <18 years |
750 |
2800 |
| >18 years |
770 |
3000 |
| Lactation | ||
| <18 years |
1200 |
2800 |
| >18 years |
1300 |
3000 |
|
Note: RDA – recommended dietary allowance AI – adequate intake, it means this level is expected to ensure nutritional adequacy and it is given where the evidence is not satisfactory to get an RDA UL – upper limit, which is the maximum daily intake that will not cause adverse effect |
||
Vitamin A Deficiency:
A plasma retinol level less than 0.70 micromol/L is considered deficiency [4]. Another indicator for vitamin A deficiency is serum retinol-binding protein (RBP).
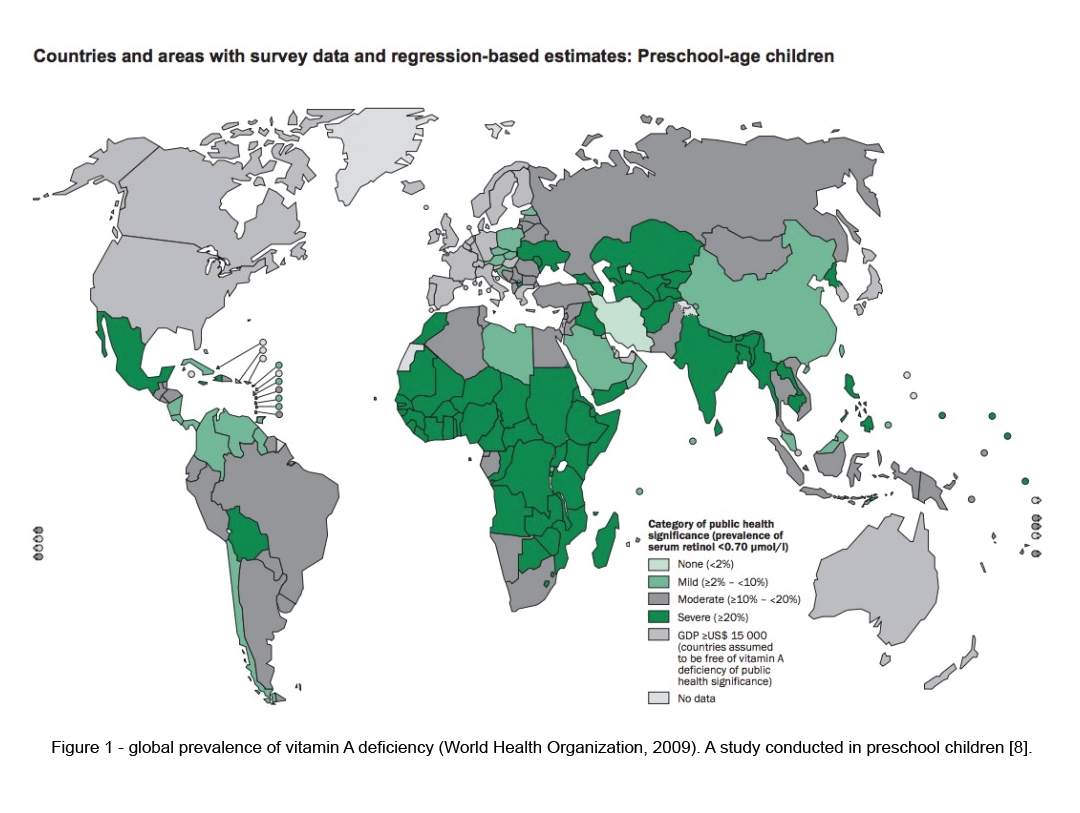
Vitamin A deficiency is relatively rare in North America. However, the prevalence is approximately between 30% – 50% in the developing world, (figure1), especially in the preschool children [13]. It is one of the leading cause for blindness in underdeveloped countries. Hence, Vitamin A supplementation is included in the vaccination schedule of children in both developing and underdeveloped countries. Studies have shown that this effort was successful in preventing vitamin A related blindness and deaths.
Vitamin A deficiency causes the disease called Xerophthalmia. The early signs include night blindness or not being able to see in the dark. Other signs are Bitot’s spots in the eye, conjunctival xerosis, corneal xerosis & ulceration, and corneal scarring leading to xeropthalmia. Other health issues include decreased bone health, hyperkeratosis, follicular hyperkeratosis, or phrynoderma, and children are prone to measles and diarrhea [14].
Causes of vitamin A deficiency include dietary deficiency which is the case in developing and underdeveloped countries. Chronic diarrhea in children is also a cause for deficiency of vitamin A in developing countries. However, in developed countries, the cause for deficiency is much different and certain populations are at high risk of deficiency, the major reason for which is malabsorption.
The risk groups for deficiency include;
- Pancreatic insufficiency
Cystic fibrosis patients develop vitamin A deficiency due to associated pancreatic insufficiency where fat absorption is affected. Several studies have shown that 15-40% of patients with cystic fibrosis develop vitamin A deficiency [9]. Cystic fibrosis patients are treated with fat-soluble vitamins at doses higher than their required daily intake. Chronic pancreatitis patients generally do not develop vitamin A deficiency as some of the pancreatic function is still preserved. Short bowel syndrome also causes vitamin A deficiency due to fat malabsorption. - Chronic liver disease
As the liver is the storehouse of Vitamin A, chronic liver disease can cause deficiency of fat-soluble vitamins like vitamin A. The metabolism of retinol and ethanol occurs through cytochrome P450 leading to increased metabolism of retinol. This combined with decreased dietary intake results in Vitamin A deficiency. However, the process is much more complicated as it can also cause hepatotoxicity when combined with ethanol [10]. - Bariatric surgery
After bariatric surgery the deficiency of vitamin A increases. By about 6 weeks of bariatric surgery 35% showed vitamin A deficiency if not supplemented [11, 12]. - Crohn’s disease (CD)
Due to extensive small bowel involvement, CD patients are at increased risk of fat-soluble vitamin deficiency. - Celiac disease
Newly diagnosed celiac disease patients might have deficiencies and may require supplementations. But once they are stable with a gluten-free diet, their requirements come back to a normal healthy individual.
Other population that might be at risk for developing vitamin A deficiency, due to inadequate intakes of vitamin A;
- Premature infants
In premature babies the liver stores for vitamin A are not sufficient as the serum levels of retinol remains low for the 1st year which makes them susceptible for deficiency related diseases [6]. - Infants and young children in developing countries
In developed or developing countries, breast milk can provide them with adequate vitamin A which will not occur if mothers are deficient of vitamin A [7]. Moreover, the incidence of vitamin A deficiency spikes once the exclusive breastfeeding stops. -
Pregnant and lactating women in developing countries
As the nutritional demands during pregnancy and lactation increases, the need for vitamin A also increases. In developing countries, WHO estimates that 9.8 million pregnant women around the world have xerophthalmia due to Vitamin A deficiency [14].
Treatment of Vitamin A Deficiency
Replacement of vitamin A is the treatment for deficiency. WHO recommends periodic supplementation of vitamin A in endemic population in the suggested doses [37]:
- 6 months – 1 year – 10,000 IU PO single dose
- 1 year – 5 year – 200,000 IU PO repeated over 4-6 months.
- Pregnant women in endemic areas should also receive supplementation – doses <10,000 IU daily
-
Children in endemic areas are at high risk of deficiency related diseases and should receive supplements if they haven’t received the routine recommended doses.
High risk measles – require 2 doses in 2 subsequent days
Xerophthalmia – 3 doses are given, 1st dose at diagnosis, 2nd the next day and 3rd dose after 2 weeks
Vitamin A Overdose:
The metabolism of plant sources of Vitamin A is highly regulated, so too much intake from dietary sources is very unlikely to cause toxicity. However, excess dietary intake from plant sources can cause yellowing of the skin called carotenemia but will not develop a toxicity.
Acute toxicity – In adults, acute toxicity develops when a single dose of >660,000 IU (>200,000 mcg) of retinol is ingested. Symptoms of acute toxicity include nausea, vomiting, loss of appetite, abdominal pain, dizziness, irritability, drowsiness, raised intra cranial pressure due to cerebral oedema, headache, desquamation, coma or even death [35].
Chronic toxicity usually occurs due to long-term injection of vitamin A which is more than 10 times the RDA [31]. The toxic effects include hepatomegaly, splenomegaly, severe headache, pseudo tumor cerebri, hair changes, alopecia, dry rough cracked skin, and lips, general weakness, arthralgia, hyperostosis of the bone and fractures by minor trauma. In children the signs and symptoms may be slightly different. It includes irritability, drowsiness, delirium, raised intra cranial pressure, bulging fontanelles, psychiatric changes, bulging eyeball, visual disturbances, skin desquamation etc. [35, 36].
Effects on bone – Another risk associated with vitamin A rich diet is the risk of fractures and osteopenia. Several studies conducted by nurses have shown that a diet high in carotenoids is associated with increased risk of fractures in post-menopausal women, and it accelerates osteoporosis [28, 29, 30, 32].
Teratogenicity – In pregnancy, higher doses can result in congenital anomalies due to the teratogenicity of vitamin A. At recommended doses, they are not teratogenic. Consumption exceeding 10,000 IU of preformed vitamin A per day during 1st trimester has caused genetic malformations [27]. The birth defects include encephalitis, microcephaly, craniofacial malformation like cleft palate, cardiovascular abnormalities including transposition of great vessels and thymus abnormalities [36].
Treatment if Vitamin A Overdose
Overdose or toxicity is treated by stopping the use of vitamin A supplements. Generally, the symptoms and signs will subside gradually in a few weeks depending on the severity. However, birth defects caused by the teratogenic effects are irreversible.
Vitamin A and Cancer:
The association between vitamin A and different types of cancers is controversial. It has shown to reduce the risk of certain cancers whereas increasing the risk of other cancers.
- Lung Cancer
Studies have shown that supplementation with carotenoids can increase the risk of lung cancer in men with other risk factors like smoking or asbestos exposure [18, 19].
- Prostate Cancer
Beta carotene supplementation in certain studies have shown an increase in mortality in the study population [20]. However, another study has shown an increase in survival of patients who took beta carotene [21].
- Colorectal cancer
In a study conducted in 864 patients by supplementing antioxidants including vitamin A, no association was found between incidence of colorectal adenoma and cancer reduction [22]. - Breast Cancer
The results of studies related with breast cancer and vitamin A intake is mixed. A study conducted among postmenopausal Iowa women concluded no association between breast cancer and vitamin A intake [23]. However, multiple other studies conducted among premenopausal women suggests that intake of vitamin A reduces the risk of breast cancer, and a diet low in vitamin A increases the susceptibility for breast cancer [24, 25, 26].
The inconsistent results between different studies and trials relating to vitamin A and cancer is owing to confounding factors like exposure to other risk factors like tobacco in many cancers.
Vitamin A and COVID-19:
The data to comment on the effectiveness of vitamin A in COVID-19 treatment and prevention is limited. However, several clinical trials have been conducted in intensive care settings and have developed several hypotheses in support of the regular use of vitamin A and other antioxidants. These studies have shown that Vitamin A, due to its antioxidant effects, immune function, role in natural barriers and the local paracrine signaling, have been effective in acute respiratory distress syndrome (ARDS) and COVID-19 [33]. Another study has also shown that Vitamin A is effective in treatment of pneumonia which makes it a good treatment option for COVID-19 [34].
Conclusion:
Vitamin A is a fat-soluble micronutrient with antioxidant properties. Even after being available in abundance, certain countries are still endemic for vitamin A deficiency. Some develop toxicity due to excessive ingestion, the worst being teratogenic effects. Vitamin A is also used in treatment of different diseases including COVID-19. Vitamin A is a micronutrient with an abundant research scope in the future. Due to the endemicity of vitamin A in certain countries, research on modifying gut bacteria to synthesize vitamin A in risk groups instead of biofortification can better manage the deficiency [44]. As the cancer and vitamin A is always a study with debates, the research on epigenetic role of vitamin A in cancer would have wide scope in the near future [5]. Vitamin A will continue to intrigue the scientific community resulting in an increase in research to further understand and develop the existing knowledge base for this nutrient.
References:
- Johnson EJ, Russell RM. Beta-Carotene. In: Coates PM, Betz JM, Blackman MR, et al., eds. Encyclopedia of Dietary Supplements. 2nd ed. London and New York: Informa Healthcare; 2010:115-20.
- Ross CA. Vitamin A. In: Coates PM, Betz JM, Blackman MR, et al., eds. Encyclopedia of Dietary Supplements. 2nd ed. London and New York: Informa Healthcare; 2010:778-91.
- Institute of Medicine: Food and Nutrition Board. (2001). Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washingto, DC:The National Academies Press, 82-162. https://doi.org/10.17226/10026
- de Pee, S., & Dary, O. (2002). Biochemical Indicators of Vitamin A Deficiency: Serum Retinol and Serum Retinol Binding Protein. The Journal Of Nutrition, 132(9), 2895S-2901S. https://doi.org/10.1093/jn/132.9.2895s
- Bar-El Dadon, S., & Reifen, R. (2015). Vitamin A and the epigenome. Critical Reviews In Food Science And Nutrition, 57(11), 2404-2411. https://doi.org/10.1080/10408398.2015.1060940
- Darlow, B., & Graham, P. (2007). Vitamin A supplementation to prevent mortality and short and long-term morbidity in very low birthweight infants. Cochrane Database Of Systematic Reviews. https://doi.org/10.1002/14651858.cd000501.pub2
- Oliveira-Menegozzo, J., Bergamaschi, D., Middleton, P., & East, C. (2010). Vitamin A supplementation for postpartum women. Cochrane Database Of Systematic Reviews. https://doi.org/10.1002/14651858.cd005944.pub2
- World Health Organization [u.a.]. (1995). Global prevalence of vitamin A deficiency.
- Borowitz, D., Baker, R., & Stallings, V. (2002). Consensus Report on Nutrition for Pediatric Patients With Cystic Fibrosis. Journal Of Pediatric Gastroenterology And Nutrition, 35(3), 246-259. https://doi.org/10.1097/00005176-200209000-00004
- Leo, M., & Lieber, C. (1999). Alcohol, vitamin A, and β-carotene: adverse interactions, including hepatotoxicity and carcinogenicity. The American Journal Of Clinical Nutrition, 69(6), 1071-1085. https://doi.org/10.1093/ajcn/69.6.1071
- Zalesin, K., Miller, W., Franklin, B., Mudugal, D., Rao Buragadda, A., & Boura, J. et al. (2011). Vitamin A Deficiency after Gastric Bypass Surgery: An Underreported Postoperative Complication. Journal Of Obesity, 2011, 1-4. https://doi.org/10.1155/2011/760695
- Kushner, R., Herron, D., Herrington, H., Jones, D., & Chen, W. (2021). Bariatric surgery: Postoperative nutritional management. UpToDate. Retrieved 22 August 2021, from https://www.uptodate.com/contents/bariatric-surgery-postoperative-nutritional-management?topicRef=2571&source=see_link#H2193490928.
- UNICEF. Coverage at a Crossroads: New directions for vitamin A supplementation programmes. New York: UNICEF; 2018. Available at: UNICEF. Coverage at a Crossroads: New directions for vitamin A supplementation programmes. New York: UNICEF; 2018.
- World Health Organization. Global Prevalence of Vitamin A Deficiency in Populations at Risk 1995–2005: WHO Global Database on Vitamin A Deficiency . Geneva: World Health Organization; 2009
- Vivekananthan, D., Penn, M., Sapp, S., Hsu, A., & Topol, E. (2003). Use of antioxidant vitamins for the prevention of cardiovascular disease: meta-analysis of randomised trials. The Lancet, 361(9374), 2017-2023. https://doi.org/10.1016/s0140-6736(03)13637-9
- Araki, S., Kato, S., Namba, F., & Ota, E. (2018). Vitamin A to prevent bronchopulmonary dysplasia in extremely low birth weight infants: a systematic review and meta-analysis. PLOS ONE, 13(11), e0207730. https://doi.org/10.1371/journal.pone.0207730
- Age-Related Eye Disease Study Research Group (2001). A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Archives of ophthalmology (Chicago, Ill. : 1960), 119(10), 1417–1436. https://doi.org/10.1001/archopht.119.10.1417
- Omenn, G. S., Goodman, G. E., Thornquist, M. D., Balmes, J., Cullen, M. R., Glass, A., Keogh, J. P., Meyskens, F. L., Valanis, B., Williams, J. H., Barnhart, S., & Hammar, S. (1996). Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. The New England journal of medicine, 334(18), 1150–1155. https://doi.org/10.1056/NEJM199605023341802
- Virtamo, J., Pietinen, P., Huttunen, J. K., Korhonen, P., Malila, N., Virtanen, M. J., Albanes, D., Taylor, P. R., Albert, P., & ATBC Study Group (2003). Incidence of cancer and mortality following alpha-tocopherol and beta-carotene supplementation: a postintervention follow-up. JAMA, 290(4), 476–485. https://doi.org/10.1001/jama.290.4.476
- Neuhouser, M. L., Barnett, M. J., Kristal, A. R., Ambrosone, C. B., King, I. B., Thornquist, M., & Goodman, G. G. (2009). Dietary supplement use and prostate cancer risk in the Carotene and Retinol Efficacy Trial. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology, 18(8), 2202–2206. https://doi.org/10.1158/1055-9965.EPI-09-0013
- Watters, J. L., Gail, M. H., Weinstein, S. J., Virtamo, J., & Albanes, D. (2009). Associations between alpha-tocopherol, beta-carotene, and retinol and prostate cancer survival. Cancer research, 69(9), 3833–3841. https://doi.org/10.1158/0008-5472.CAN-08-4640
- Greenberg, E. R., Baron, J. A., Tosteson, T. D., Freeman, D. H., Jr, Beck, G. J., Bond, J. H., Colacchio, T. A., Coller, J. A., Frankl, H. D., & Haile, R. W. (1994). A clinical trial of antioxidant vitamins to prevent colorectal adenoma. Polyp Prevention Study Group. The New England journal of medicine, 331(3), 141–147. https://doi.org/10.1056/NEJM199407213310301
- Kushi, L. H., Fee, R. M., Sellers, T. A., Zheng, W., & Folsom, A. R. (1996). Intake of vitamins A, C, and E and postmenopausal breast cancer. The Iowa Women’s Health Study. American journal of epidemiology, 144(2), 165–174. https://doi.org/10.1093/oxfordjournals.aje.a008904
- Hunter, D. J., Manson, J. E., Colditz, G. A., Stampfer, M. J., Rosner, B., Hennekens, C. H., Speizer, F. E., & Willett, W. C. (1993). A prospective study of the intake of vitamins C, E, and A and the risk of breast cancer. The New England journal of medicine, 329(4), 234–240. https://doi.org/10.1056/NEJM199307223290403
- Zhang, S., Hunter, D. J., Forman, M. R., Rosner, B. A., Speizer, F. E., Colditz, G. A., Manson, J. E., Hankinson, S. E., & Willett, W. C. (1999). Dietary carotenoids and vitamins A, C, and E and risk of breast cancer. Journal of the National Cancer Institute, 91(6), 547–556. https://doi.org/10.1093/jnci/91.6.547
- Tamimi, R. M., Hankinson, S. E., Campos, H., Spiegelman, D., Zhang, S., Colditz, G. A., Willett, W. C., & Hunter, D. J. (2005). Plasma carotenoids, retinol, and tocopherols and risk of breast cancer. American journal of epidemiology, 161(2), 153–160. https://doi.org/10.1093/aje/kwi030
- Rothman, K. J., Moore, L. L., Singer, M. R., Nguyen, U. S., Mannino, S., & Milunsky, A. (1995). Teratogenicity of high vitamin A intake. The New England journal of medicine, 333(21), 1369–1373. https://doi.org/10.1056/NEJM199511233332101
- Melhus, H., Michaëlsson, K., Kindmark, A., Bergström, R., Holmberg, L., Mallmin, H., Wolk, A., & Ljunghall, S. (1998). Excessive dietary intake of vitamin A is associated with reduced bone mineral density and increased risk for hip fracture. Annals of internal medicine, 129(10), 770–778. https://doi.org/10.7326/0003-4819-129-10-199811150-00003
- Feskanich, D., Singh, V., Willett, W. C., & Colditz, G. A. (2002). Vitamin A intake and hip fractures among postmenopausal women. JAMA, 287(1), 47–54. https://doi.org/10.1001/jama.287.1.47
- Michaëlsson, K., Lithell, H., Vessby, B., & Melhus, H. (2003). Serum retinol levels and the risk of fracture. The New England journal of medicine, 348(4), 287–294. https://doi.org/10.1056/NEJMoa021171
- Myhre, A. M., Carlsen, M. H., Bøhn, S. K., Wold, H. L., Laake, P., & Blomhoff, R. (2003). Water-miscible, emulsified, and solid forms of retinol supplements are more toxic than oil-based preparations. The American journal of clinical nutrition, 78(6), 1152–1159. https://doi.org/10.1093/ajcn/78.6.1152
- Penniston, K. L., & Tanumihardjo, S. A. (2006). The acute and chronic toxic effects of vitamin A. The American journal of clinical nutrition, 83(2), 191–201. https://doi.org/10.1093/ajcn/83.2.191
- ‘Jovic, T. H., Ali, S. R., Ibrahim, N., Jessop, Z. M., Tarassoli, S. P., Dobbs, T. D., Holford, P., Thornton, C. A., & Whitaker, I. S. (2020). Could Vitamins Help in the Fight Against COVID-19?. Nutrients, 12(9), 2550. https://doi.org/10.3390/nu12092550
- Li, R., Wu, K., Li, Y., Liang, X., Tse, W., Yang, L., & Lai, K. P. (2020). Revealing the targets and mechanisms of vitamin A in the treatment of COVID-19. Aging, 12(15), 15784–15796. https://doi.org/10.18632/aging.103888
- Pazirandeh, S., Burns, D., Seres, D., Motil, K., & Kunins, L. (2021). Overview of Vitamin A. UpToDate. Retrieved 23 August 2021, from https://www.uptodate.com/contents/overview-of-vitamin-a?source=history_widget#H15.
- Wu, B., & Oakley, A. (2015). Vitamin A toxicity. DermNet NZ: All About Skin. Retrieved 22 August 2021, from https://dermnetnz.org/topics/vitamin-a-toxicity/#:~:text=Signs%20and%20symptoms%20of%20acute%20vitamin%20A%20toxicity,or%20desquamation%20%28peeling%20skin%29%204%20Coma%20and%20death.
- World Health Organization Guideline: Vitamin A supplementation for infants and children 6-59 months of age (2011). Available at: http://www.who.int/nutrition/publications/micronutrients/guidelines/vas_6to59_months/en/.
- Zile, M. H., & Cullum, M. E. (1983). The Function of Vitamin A: Current Concepts. Proceedings of the Society for Experimental Biology and Medicine, 172(2), 139–152. https://doi.org/10.3181/00379727-172-41537
- U.S. Department of Agriculture (USDA), Agricultural Research Service. FoodData Central: Foundation Foods. Version Current: April 2021. https://fdc.nal.usda.gov
- Booth, S., Johns, T., & Kuhnlein, H. (1992). Natural Food Sources of Vitamin A and Provitamin A. Food And Nutrition Bulletin, 14(1), 1-15. https://doi.org/10.1177/156482659201400115
- Huiming, Y., Chaomin, W., & Meng, M. (2005). Vitamin A for treating measles in children. The Cochrane database of systematic reviews, 2005(4), CD001479. https://doi.org/10.1002/14651858.CD001479.pub3
- Orfanos, C. E., Zouboulis, C. C., Almond-Roesler, B., & Geilen, C. C. (1997). Current use and future potential role of retinoids in dermatology. Drugs, 53(3), 358–388. https://doi.org/10.2165/00003495-199753030-00003
- Chomienne, C., Fenaux, P., & Degos, L. (1996). Retinoid differentiation therapy in promyelocytic leukemia. FASEB journal : official publication of the Federation of American Societies for Experimental Biology, 10(9), 1025–1030. https://doi.org/10.1096/fasebj.10.9.8801163
- Srinivasan, K., & Buys, E. (2019). Insights into the role of bacteria in vitamin A biosynthesis: Future research opportunities. Critical Reviews In Food Science And Nutrition, 59(19), 3211-3226. https://doi.org/10.1080/10408398.2018.1546670
